
By Luca Masella, M.S.
Reading Time: 4 Minutes

What is air composed of?
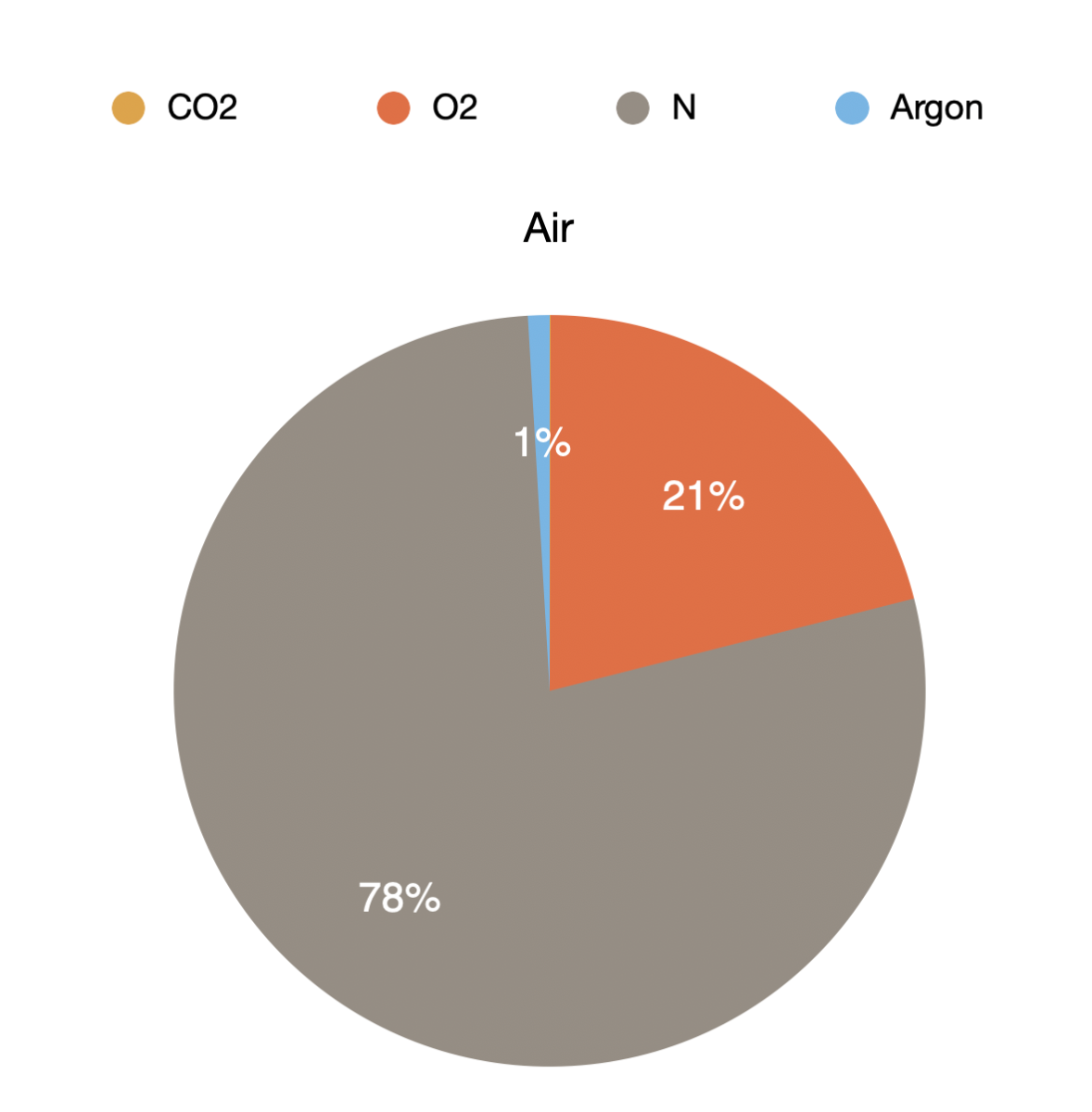
The air within our atmosphere is made up of a mixture of gases. The most relevant are:
- Oxygen 21%
- Nitrogen 78%
- Argon 0.93%
- Carbon dioxide 0.04% (400ppm)
Importance of Oxygen
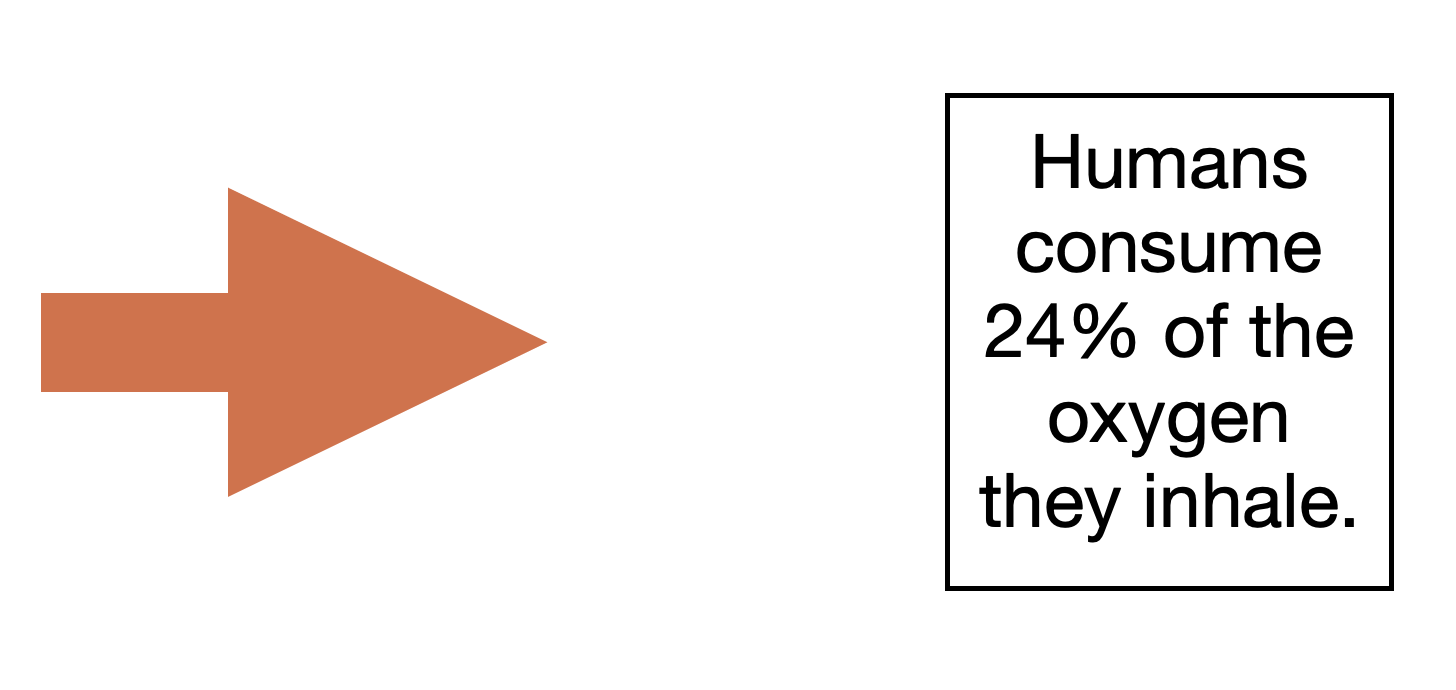
The role of respiration is to provide body cells with energy in order to support metabolism. The cells convert oxygen into ATP (adenosine triphosphate), which is the energy cells use.
Human beings inhale air and take it into the lungs where the oxygen is then separated. The air mixture then comes back with the byproduct of cell metabolism, which is Co2. That is the reason why we:
How much space does a person need to breath without affecting the Co2 levels?
The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) has developed ventilation guidelines to maintain a comfortable environment for most occupants. In office spaces, ASHRAE recommends 17 cubic feet per minute for each person. In addition, Minnesota Department of Labor and Industry (MNDOLI) Rule states that "outside air shall be provided to all indoor workrooms at the rate of 15 cubic feet per minute per person (MN Rule, 5205.110)”.
Based on this, it could be stated that by providing 200 square feet per person, throughout the whole working day, each person would be able to breath without any problems.
These rules must be followed to keep the carbon dioxide concentrations below 1,000 ppm. However, it’s challenging to maintain these levels since offices are usually maintained at a much higher occupancy and the air change rate is lower, considering it is only operated by an air conditioning system, which generally lack adjustable fresh air intake. Therefore, it’s essential to keep track of Co2 concentration with sensors.
It is difficult to quantify the effects of long-term exposure to higher levels of Co2 due to a lack of long term research. To demonstrate effects, we could compare it to the effects of poisonous substances.
Case Study 1: Lack of oxygen in closed room overnight
Consider a small (10’x10’x8’) closed room (no air coming in from outside) which contains 800 cubic feet of air. Note that the 800 cubic feet of air is not just oxygen, but the air mix therefore it is just 21% oxygen, which is actually just 168 cubic feet.
The average adult at rest inhales, on average, 550 liters (145 gallons) a day of pure oxygen; (550 liters -> 19 cubic feet). With this in mind, a human being could hypothetically survive almost 9 days in this airtight room.
The main area of concern is the Co2 concentration. Since humans exhale 5% of carbon dioxide, it is really easy to get to a significant amount (over 1000 ppm).
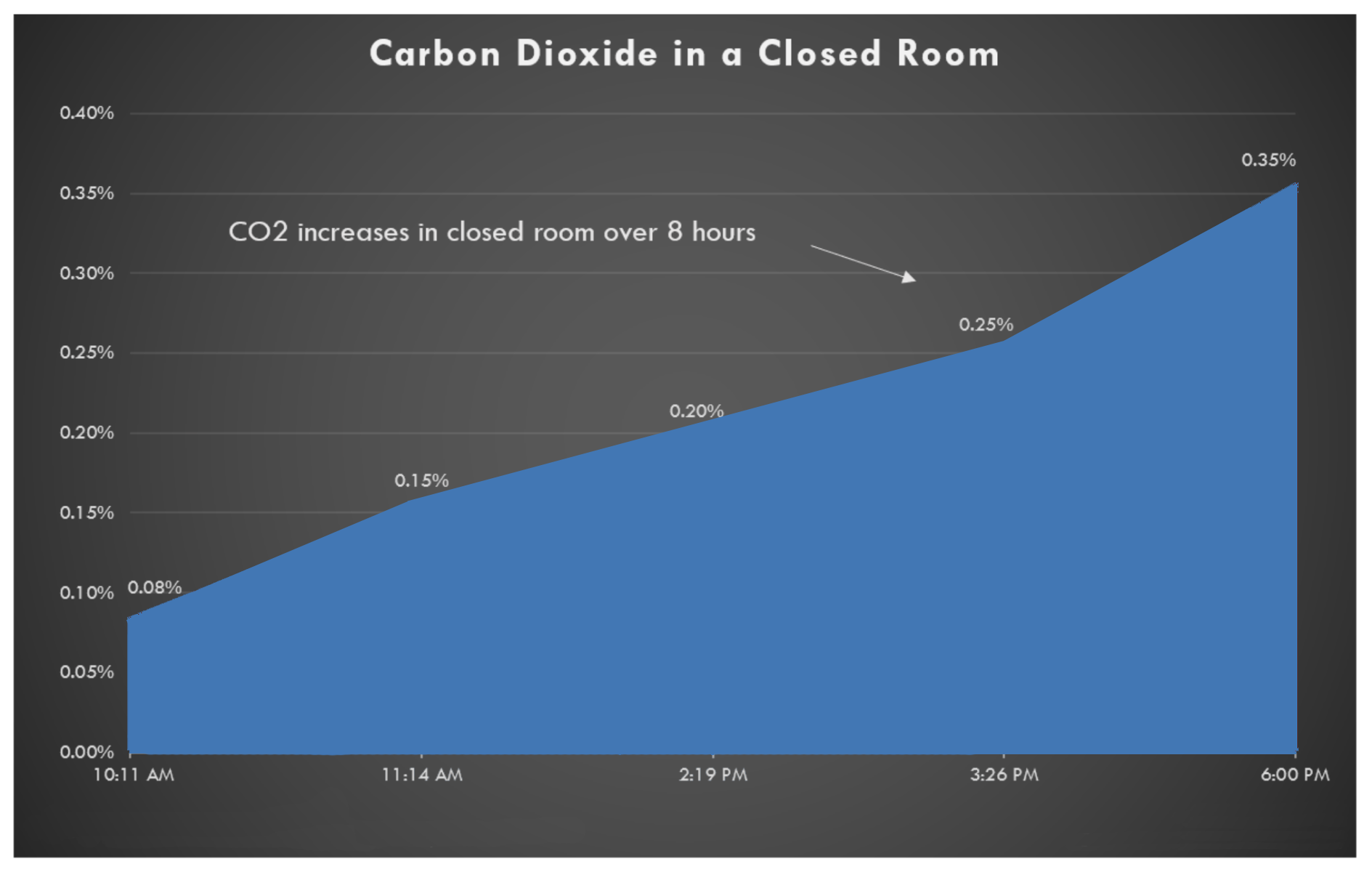
In the closed room, the Co2 increases by 0.05% (500ppm) per hour which means after an hour a person’s cognitive functions would decrease by 20%. After 40 hours the level of Co2 gets to 20,000 ppm (2%) at which point one would start experiencing shortness of breath.
It is important to note that the air mix contains a much higher concentration of oxygen than Co2, so it is very easy to produce large increments of Co2 while maintaining a small variation in oxygen levels.
A high concentration of oxygen in the air mix is necessary to properly feed the body’s cells, so a small decrease in oxygen due to the transformation of O2 in Co2 can have a dangerous effect on the human body. It is not necessarily easy to run out of oxygen in a room, but one can still suffer from asphyxiation by hypoxia (too much Co2 in a room). When one breathes it in, carbon dioxide prevents the blood cells from carrying enough oxygen. The brain and heart suffer quickly, and while the body can inform itself if it lacks oxygen, it cannot detect a lack of Co2.
Part 2 - Coming Soon





