
By Luca Masella, M.S.
Reading Time: 5:45 Minutes

How to reduce CO2
There are a number of possible solutions to deal with higher CO2 levels indoors.
1. Higher fresh air intake — this works only if outdoor CO2 levels are low in that area, and is a short term solution.
This method tends to use more energy, but as long as there is clean air outside, bringing it inside is the easiest way to lower indoor CO2 concentrations.
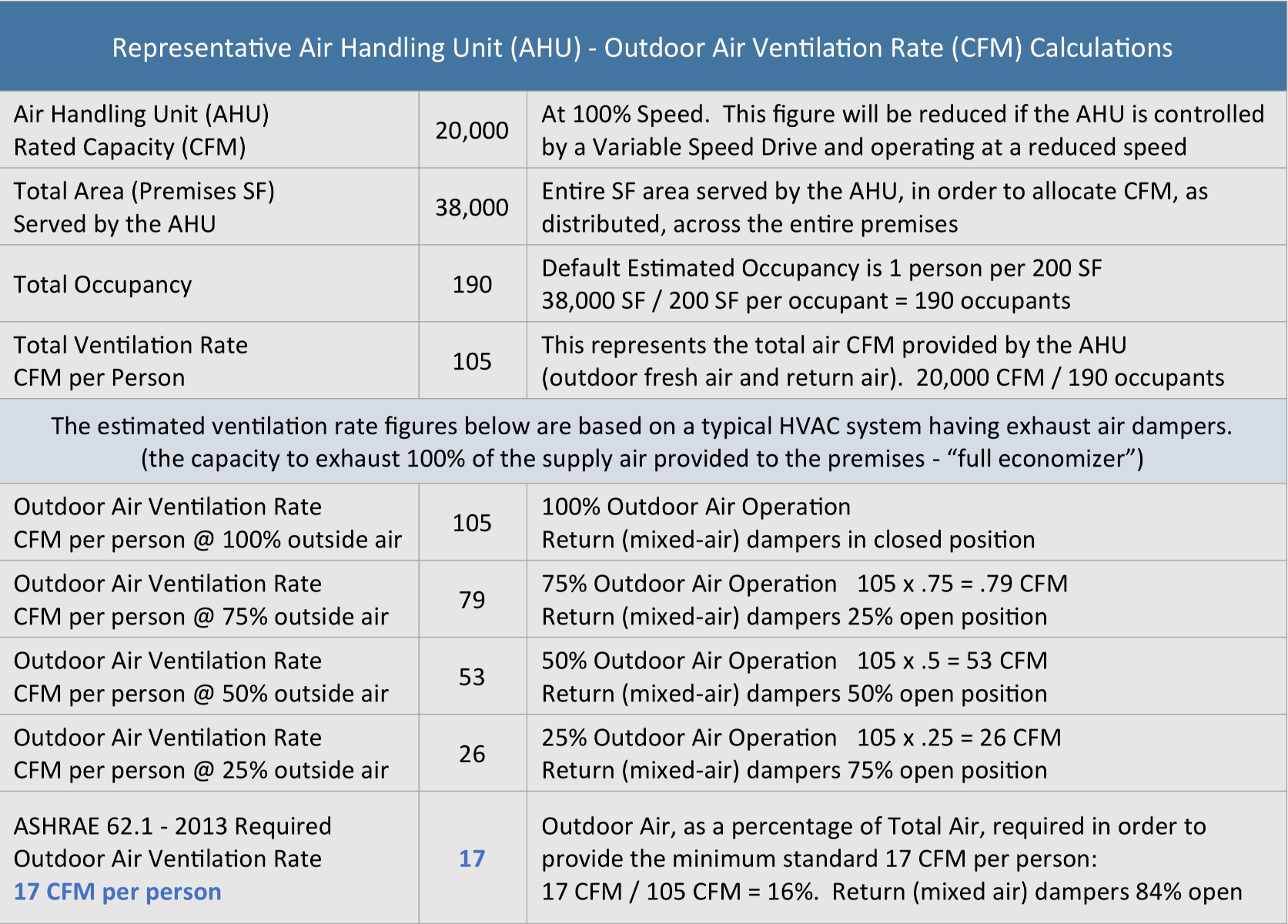
HVAC systems, mostly recirculate existing air while only bringing in a small percentage of fresh air. According to ASHRAE recommendations, occupancy in office spaces must fall under 200 sf per person and 17 CFM (cubic feet per minute) of fresh air per person.
The chart below shows an example of a 38,000 sf building with an average HVAC system, which blows at about 20,000 CFM which is 105 CFM per person following the occupancy recommendations. Consider there are 190 occupants inside. Since the recommendation is to achieve 17 CFM of fresh air out of the 105 CFM per person, the solution would be to introduce a mix with 16% fresh air from outside.
To improve the thermal comfort level, such as by lowering the air throw (air throw is the distance from the ac vent to a point where the velocity of the air stream ends to a specified velocity) and noise made by the HVAC system, one could lower the ventilation rate from 105 CFM to 60 CFM. However at that level one would need 28% (17 CFM of fresh air over 60 total CFM = 28%) fresh air to meet the 17 CFM per person ASHRAE recommendation. However that would be inefficient and costly as you would need to re-cool or re-heat that 28% of fresh air instead of merely 16%.
ASHRAE recommendations also sets 5,000 ppm as the limit for CO2 concentration in a working space.
2. Plant or bring in more potted trees and/or other plant life
This is the most effective way, since plants naturally improve air quality by taking in CO2 and through photosynthesis, produce oxygen.

According to research done at UC Berkley, there is evidence that as global CO2 levels have increased, the terrestrial biosphere has been responding by taking in more CO2 and reducing the rate of growth of global CO2. [https://www.CO2meter.com/blogs/news/could-global-CO2-levels-be-reduced-by-planting-trees] A human being produces 1.98 lbs of CO2 everyday (725 lbs per year). A large tree consumes 48 lbs CO2 a year, which means 15 large trees are needed in order to consume all the CO2 produced by a single person annually.
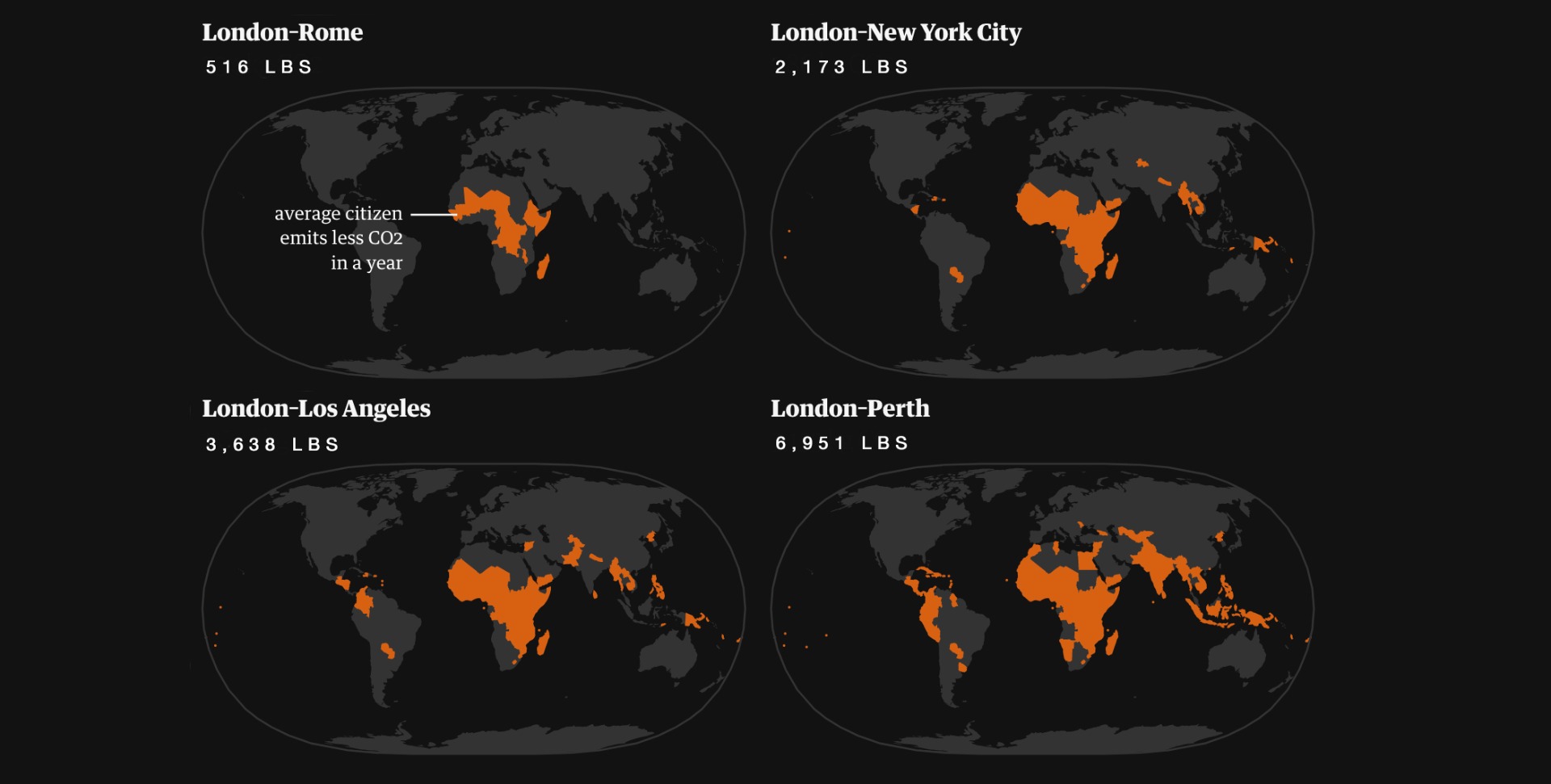
To absorb what a single person produces in a day, 2690 sqft of forest would be needed. To put this into perspective, a single flight from London to Rome produces nearly the same quantity of CO2 (515 lbs) as a single person produces in a year (725 lbs). The populations represented by the areas marked in orange below, compare the amount of CO2 produced by one flight with how much the population emits in a year
While indoor plants may not solve planetary issues with CO2 levels, they do help improve the quality of air for those staying indoors for long periods of time. As an example, 54 sqft is the typical size for an indoor living wall. 54 sqft of living wall extracts 25.3 lbs of CO2 per year and produces 18.7 lbs of oxygen. This is nothing compared to what a single person produces, but it is still helpful to reduce the indoor concentrations.
Additionally, plants absorb sunlight: 50% is absorbed and 30% reflected, creating a cooler and more pleasant climate. For indoor climate, this means 33% less air conditioning is required, allowing for more energy savings.

3. Use building material with low permeability (the state or quality of a material or membrane that causes it to allow liquids or gases to pass through it.) to CO2
A physics research group at Tampere University of Technology looked into the carbon dioxide permeability of building materials. Ideally, building materials can filter the air to keep the CO2 from entering buildings. Common plastics, for example, allow over a hundred times more CO2 to escape than others.
A Middle East Technical University thesis compared the behavior of red bricks and AAC bricks (autoclave aerated concrete) with CO2. For building materials, what is desired is a permeable skin so the building can breath and expel the CO2. Their study revealed that a certain portion of that CO2 retainment is due to the reactions between the minerals in AAC bricks and CO2.
When the air temperature increases, the CO2 adsorption (molecule adhere to a surface) capacity of AAC bricks increases. In short, a considerable amount of CO2 in the air can be retained inside by AAC bricks.
On the other hand the red bricks do not keep the CO2. The porous structure of red bricks allows humidity and CO2 transmission from one side to the other side while the porous structure of AAC brick material allows humidity transmission through its material, but it does not permit the transmission of CO2 from one side to the other due to its chemical reaction with CO2
4. A future solution is the production of artificial photosynthesis
Artificial photosynthesis aims to store renewable energy in chemical bonds spanning fuels, foods, medicines, and materials using light, water, and CO2 as the primary chemical feedstocks. In 2019, researchers working with Professor Erwin Reisner of Cambridge University’s Department of Chemistry developed a solar reactor based on an ‘artificial leaf’ design, which also uses sunlight, carbon dioxide and water to produce a fuel, known as syngas (fuel gas mixture consisting primarily of hydrogen and is used to produce a range of commodities, such as fuels, pharmaceuticals, plastics and fertilizers).

While eliminating CO2 to produce energy has not been developed yet, research is moving in that direction. In the near future, we will be able to solve two issues at the same time: reducing CO2 and providing an alternative energy source.
5. CO2 capture technology
A modern technology called “electro-swing cell” could be a viable solution. When the system’s temperature is lowered (or pressure is increased), CO2 adheres (sticks to) to the sorbent (a substance which can collect molecules of anther substance) surface. When the temperature is raised (or pressure reduced), the CO2 is released. But completing temperature or pressure cycles consumes a significant amount of energy.
Ralph Landau, Professor of Chemical Engineering and co-director of MIT, focused his research on a family of molecules called quinones. When these molecules are forced to became negatively charged, they have a high chemical attraction for CO2 molecules and grab any that pass. When the extra electrons are then removed, the quinone’s chemical affinity for CO2 dissipates, and the molecules release the CO2. It is possible to capture CO2 just by charging batteries. This solution could be important in some indoor applications in preventing energy waste from the HVAC having to reheat or re-cool the air taken from outside by filtering out the CO2 from the indoor air. The ideal applications for this technology would be environments in extreme cold or hot locations or in areas of heavy outside pollution.
6. Direct air capturing
The DAC (Direct Air Capturing) method uses chemical reactions to absorb CO2. When air moves over the chemicals, they react with and remove CO2. This allows the other components of air to pass through. The CO2 is then stored underground in permanent storage or used in multiple products and applications. Permanent storage would benefit the climate the most over other solutions. Eliminating the CO2 from the air and transforming would be a definitive solution.

Fun Facts: CO2 on other planets
Carbon Dioxide is also found on other planets. For example, Venus’ atmosphere consists of roughly 96 – 97% carbon dioxide. Because of the large amount of carbon dioxide present, the surface of Venus continually retains heat and, as such, the surface temperature is roughly 467°C, making it the hottest planet in our solar system.
On Mars, Carbon dioxide has been found in snow. In fact, the atmosphere is made by 96% of carbon dioxide, the remaining is mostly argon and nitrogen. Like Earth, Mars has two permanent polar ice caps. The ones on Mars are mainly water ice, but also contain a large amount of frozen carbon dioxide. In fact, 25-30% of Mars’ carbon dioxide is frozen at the poles.





